Abstract
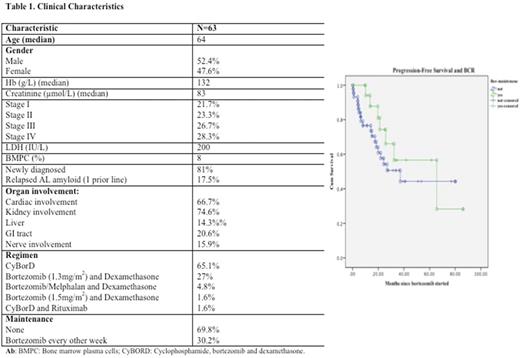
Introduction AL amyloidosis is a clonal plasma cell disorder characterized by the deposition of monoclonal light-chain fibrils into extracellular tissues, leading to organ dysfunction. Data on maintenance in AL amyloidosis is scarce and only a small study with high-dose dexamethasone followed by maintenance dexamethasone/interferon is available in the medical literature. Based on the above mentioned, we aimed to explore the role of bortezomib maintenance for the treatment of AL amyloidosis. Methods All consecutive AL amyloid patients treated at Tom Baker Cancer Center with bortezomib-containing regimens (BCR) from 01/09 to 03/17 were evaluated. A p value of <0.05 was considered significant. Survival curves were constructed according to the Kaplan-Meier method and compared using the log rank test. All statistical analyses were performed by using the SPSS 24.0 software. Results 63 consecutive patients with AL who had received BCR at our Institution over the defined period were evaluated. Clinical characteristics are shown in Table 1. 19 patients had received maintenance bortezomib (bortezomib 1.3-1.5 mg/m2 every other week) and 10 out of these 19 received more than 12 months of therapy. Median bortezomib dose on the maintenance group was 42.9 mg/m2 compared to 15.6 mg/m2 in the no-maintenance group (p=0.03). At the time of analysis, 43 patients are still alive and 14 have already progressed. No differences with regards to best response rates were seen between the maintenance and no-maintenance groups (VGPR/CR rate of 68.4% vs 63.6%, p=0.2). Further, overall survival was similar between patients receiving or not bortezomib maintenance (NR vs 72 months, p=0.6) However, a trend towards better PFS was observed for the group receiving maintenance (65 vs 37 months, p=0.3) (Fig 1) In conclusion, BCR are efficacious for the treatment of AL amyloidosis. The present study reports on the use of bortezomib maintenance for the treatment of AL patients. Our study failed to identify a significant benefit on response rates or survival but further investigation is needed to assess whether a subgroup of patients might benefit from this strategy, especially now that novel tools of monitoring such as Minimal Residual Disease assessment are investigated.
Jimenez-Zepeda: Celgene: Honoraria; Takeda: Honoraria; Amgen: Honoraria; Janssen: Honoraria. Neri: Janssen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding. Bahlis: Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal